
If you don’t understand how they are distributed in atom, then understanding any other concept in this subject is difficult. Why is it important to know how electrons are distributed in an atom for chemistry purposes?Įlectrons are the backbone of all chemistry. As each new shell is filled, electrons will drop to the next lower energy level. The shells are occupied one level at a time first shell (K), second shell (L), third shell (M) etcetera until you reach nine where there is no further Shells.
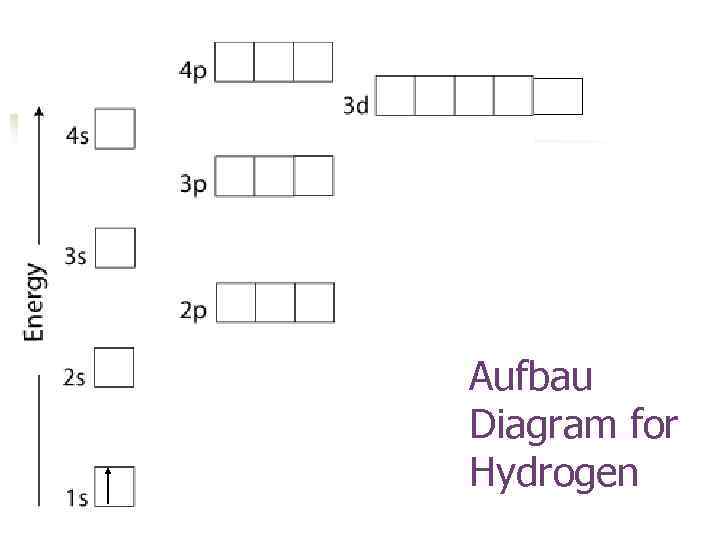

Electron configuration aufbau principle how to#
How to write Electronic Configurations?.
The importance of understanding electron configurations and their effect on chemical properties.Why is it important to know how electrons are distributed in an atom for chemistry purposes?.What are the electronic configurations of elements in the periodic table?.


 0 kommentar(er)
0 kommentar(er)
